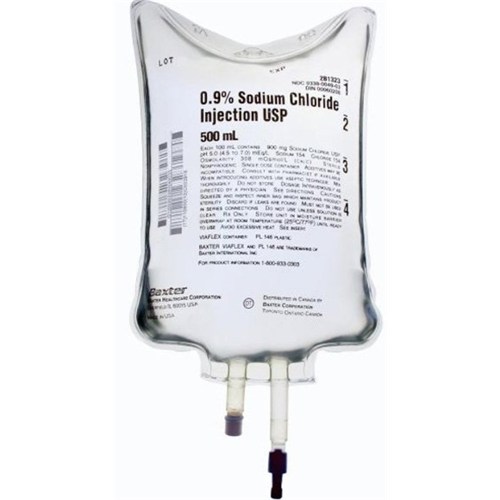
Injection Bag Sodium Chloride 0.9% Preservative Free IV Solution 500ml
Product Description
About this product
Item #:
2B1323Q
Highlights
Sodium Chloride Injection, 0.9%, USP, 500 ml, Viaflex Plastic Container, 24/cs (50 cs/plt) (Rx) (Continental US Only, Excluding IN, ND and WY) (Product Access Restricted. Check with your sales rep to verify eligibility)
- Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers for intravenous administration
- Sodium Chloride Injection, USP is indicated as a source of water and electrolytes
- It contains no antimicrobial agents
- The pH is 5.0 (4.5 to 7.0)
- 0.9% Sodium Chloride Injection, USP contains 9 g/L Sodium Chloride, USP (NaCl) with an osmolarity of 308 mOsmol/L (calc)
- The VIAFLEX plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic)
- Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million
Specifications
Item Specifications
-
Replacement Preparation
-
Flexible Bag
-
United States
-
IV Solution
-
02962
-
Sodium Chloride, Preservative Free
-
Not Made with Natural Rubber Latex
-
Baxter
-
2B1323Q
-
00338004903
-
0.9%
-
Intravenous
-
51191602
-
500 mL